
The COVID-19 vaccine has been at the forefront of the country’s collective mind. Last year, Sanguine surveyed its community of individuals that had been diagnosed with COVID-19. Those results can be found in our COVID-19 Research Participant Findings and COVID-19 Research Participant Findings Part 2 blog posts. Individuals responded to questions about their COVID-19 experience and symptoms.
Sanguine’s COVID-19 efforts have progressed since then as Pfizer’s phase 1 clinical trial findings were published in the New England Journal of Medicine. In March, we decided to reach out to our community again to gain a better understanding of individuals’ experiences with the COVID-19 vaccine.
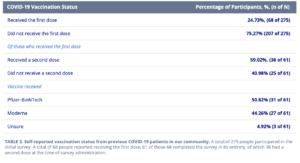
About 25% of 275 survey respondents had received the first dose of the vaccine. Of those who had received the first dose, about 60% had received the second dose as well. It seems a slight majority received the Pfizer COVID-19 vaccine rather than Moderna.
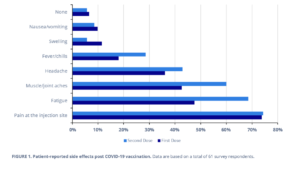
Moreover, those who have received the first and second vaccinations were also surveyed for side effects. Most respondents reported experiencing at least one side effect after receiving each dose, with a comparable portion of people having pain at the injection site (~74%), nausea and vomiting (~9%), or no symptoms (~6%) with either dose (Figure 1). The number of respondents stating fatigue, fever and chills, and muscle and joint aches was approximately one-and-a-half times higher after the second dose when compared with the first. Finally, almost two times as many people noted swelling after the first vaccination compared with the second (Figure 1).
 As we continue to grapple with COVID-19 and the varying strains to come, exploring health status from the patient’s perspective is vital in vaccine research and development. It is reassuring to learn that many individuals have been vaccinated or plan to become vaccinated soon. We hope that despite side effects, the COVID-19 vaccines can lead us into 2022 successfully and in full health.
As we continue to grapple with COVID-19 and the varying strains to come, exploring health status from the patient’s perspective is vital in vaccine research and development. It is reassuring to learn that many individuals have been vaccinated or plan to become vaccinated soon. We hope that despite side effects, the COVID-19 vaccines can lead us into 2022 successfully and in full health.